|
ALL ABOUT FUEL CELLS
Please use our A-Z INDEX to navigate this site where page links may lead to other sites
|
|
|
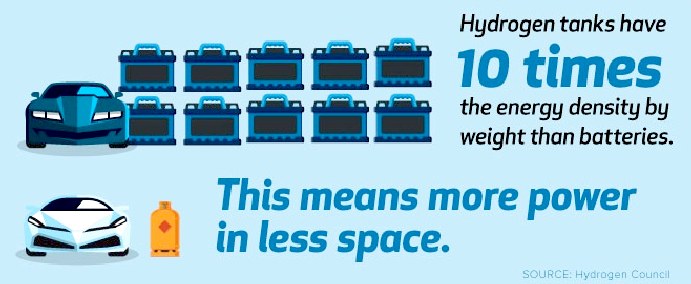
Though the energy density is greater for hydrogen, you then need to add the weight of a fuel cell stack, which detracts from the apparent energy density. Batteries include the conversion mechanism.
Hydrogen powered vehicles offer particulate free transport for cities where diesel engines are choking the population and causing lung cancer. There is an abundance of clean wind and solar energy that can produce green hydrogen, something that at the moment is not happening, but will accelerate as EVs become cheaper.
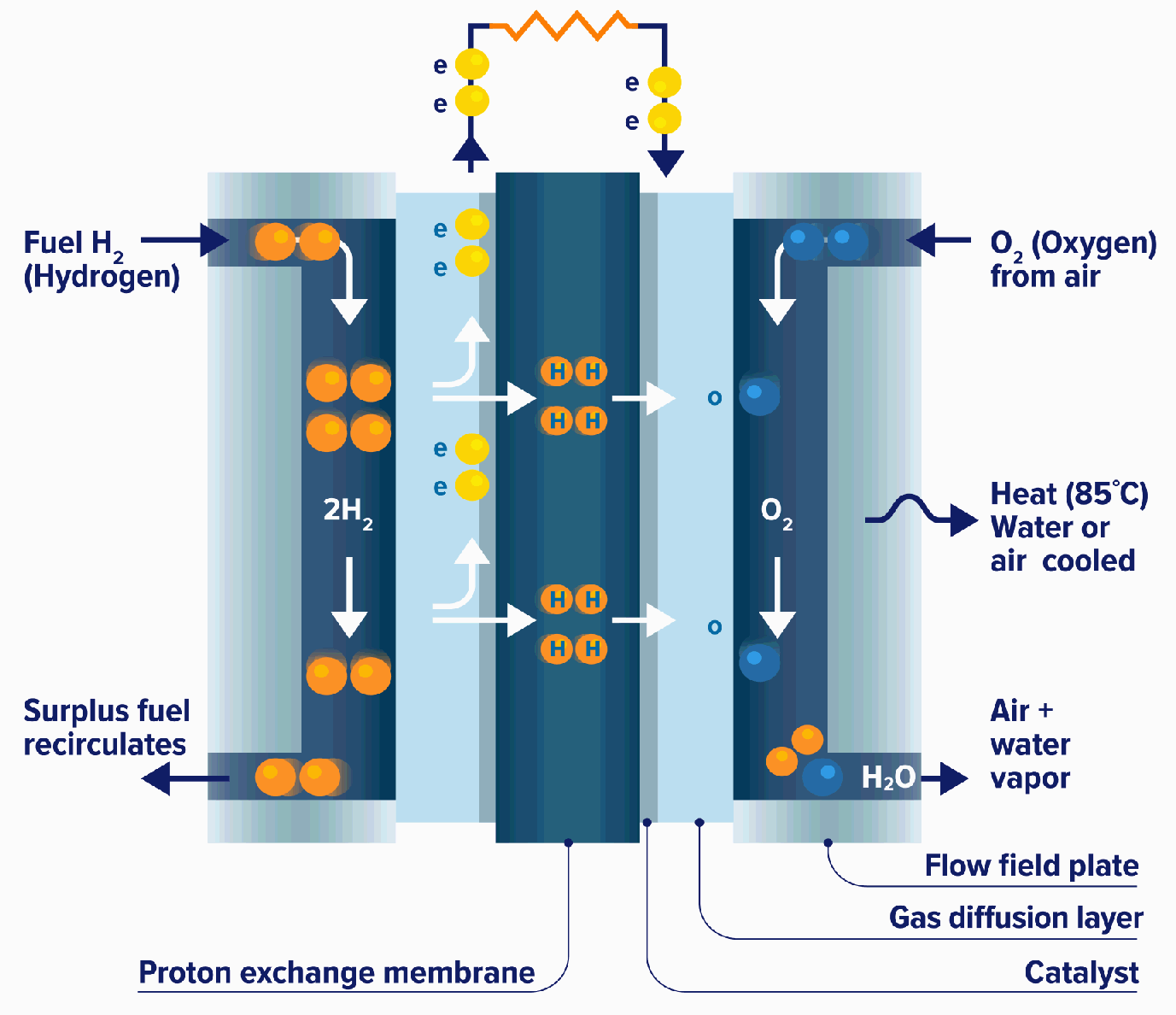
All fuel cells have a similar configuration, an electrolyte and two electrodes, but there are different types of fuel cells based mainly on what electrolyte they use.
There are six main types of fuel cells:
- PEM (proton exchange membrane),
- DMFC (direct methanol fuel cell),
- MCFC (molton carbonate fuel cell),
- PAFC (phosphoric acid fuel cell),
- SOFC (solid oxide fuel cell) and
- AFC (alkaline fuel cell).
The most popular technology is the proton exchange membrane fuel cell due to its versatility, durability, and use for a range of applications.
A fuel cell “stack” is made up of single fuel cells layered together. Depending on the application, the fuel cell stack may contain hundreds of individual cells layered together – this scalability allows fuel cells to be configured into a wide array of sizes to fit the required amount of energy desired, whether it be a ship, train, car or truck.
The electricity produced by a
fuel cell then powers a traction motor to drive a vehicle’s
wheels or propellers, or whatever device you may be powering.
At the anode, hydrogen is separated into ions and electrons using platinum or similar catalyst. The electrons travel through an external circuit, generating the required amount of power, while the ions pass through the electrolyte to the cathode where, with the help of another catalyst, they join with oxygen atoms to produce water.
TRANSPORT
If we want a practical solution to begin within the next 10 years, in 2030, hydrogen has many obstacles to overcome, where battery exchange recharging by swapping packs is already making headway in China and India, and is sure to become more popular as a way of instantly recharging EV's and also load levelling generation from solar and wind electricity.
One potential solution under development is a Dual Fuel service station where energy packs can include ammonia, hydrogen and methanol as the storage medium. With such a system, instead of competing with batteries, hydrogen interests can work alongside battery concerns to build a comprehensive transport infrastructure.
Please use our A-Z INDEX to navigate this site
This website is provided on a free basis to promote zero emission transport in Europe and Internationally. Copyright © Climate Change Trust 2022. Solar Studios, BN271RF, United Kingdom.
|